The role of regulatory bodies governing animal research

The use of live vertebrate animals (rodents, primates, birds, agricultural livestock, marine animals, wildlife, native animals, etc.) for experiments requires relevant approvals from regulatory bodies of respective governments and the university or institution. These approvals help ensure that the animals used in a research study are given humane treatment and handled with respect and care as well as to establish consistent animal care policies and standards of care for scientific research.
In each country, central bodies have been set up to ensure that researchers follow consistent and ethical animal research standards. Each country has its own set of guidelines and even though these regulations may vary slightly among countries, it is the governments that issue the rules and regulations for assuring quality maintenance and safety of laboratory animals. The ultimate aim of these guidelines is to promote good quality scientific (biomedical and behavioural) research that is conducted keeping the animals’ well-being in mind.
Does a single regulatory body to monitor animal research across the globe?
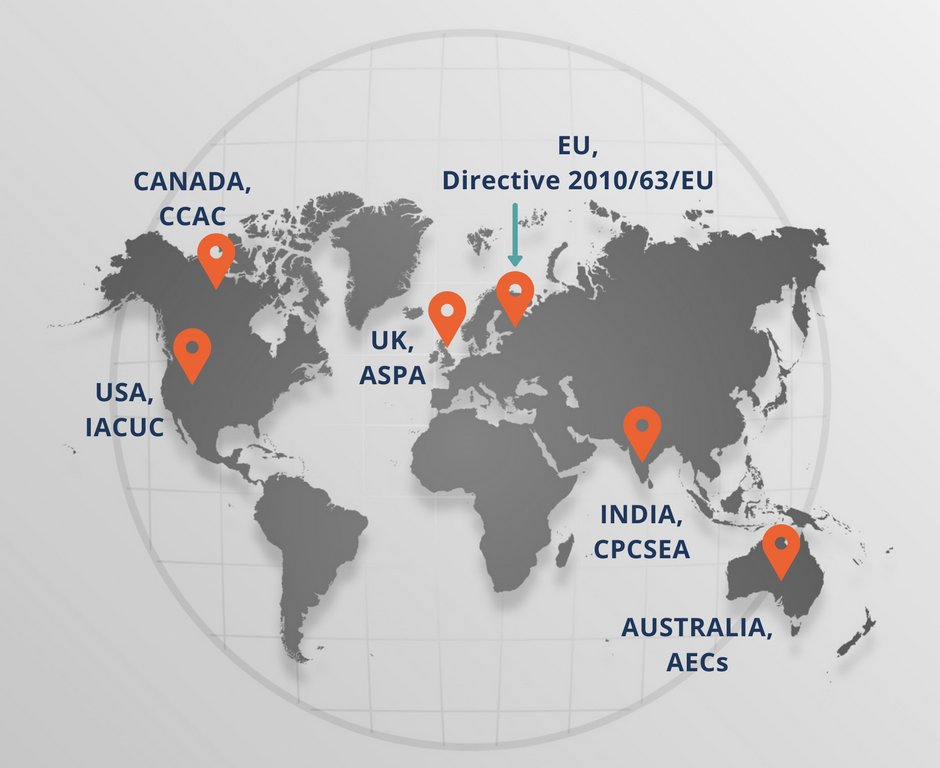
No! Each country has its own regulatory body with its own set of rules and regulations. In the US, for example, the Institutional Animal Care and Use Committee (IACUC) controls the use of animals in federally funded research institutions. Similarly, the Canadian Council on Animal Care (CCAC) assesses the use of animals in research. Directive 2010/63/EU on the protection of animals used for scientific purposes regulates the use of laboratory animals in countries belonging to the European Union. The use of animals in experiments and testing is regulated under the Animals (Scientific Procedures) Act 1986 (ASPA) in the UK. Animal Ethics Committees (AECs) in Australia determine the validity of animal usage for scientific purposes. While these are federal government bodies, each institution conducting experiments on or using animals should be certified by the respective government. The Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) set up under the aegis of the Ministry of Environment, Forest and Climate change, Government of India, is the statutory body that sets up regulatory guidelines for the use and care of laboratory animals. Each institution in India that uses animals for research must have an Institutional Animal Ethics Committee (IAEC) that handles and evaluates applications for experiments involving the use of animals and gives permission/approvals to the researchers to conduct such experiments. IAECs are local bodies and follow the guidelines formulated by the CPCSEA. Ideally, a university/research institute should also form a governing body for granting ethical approvals to individual researchers.
What does a local or institutional animal research regulatory body do?
The members of each local/institutional IAEC usually comprise a chairman/chief executive officer (these designations could vary across countries), a veterinarian, scientists with substantial recent experience in animal-based research or teaching, a member nominated by a federal regulatory body (e.g., CPCSEA, in India), a non-scientist or a person from an animal welfare group/a lay person who is socially aware and has been selected to represent the general community and does not have a formal connection to the university (whose role is to contribute different and independent perspectives to the committee's discussions and policies). Again, the constitution of these regulatory bodies is bound to change across countries.
This committee is responsible for:
- Conducting periodic evaluations/reviews of the institution's animal care facility
- Reviewing research protocols
- Monitoring and reporting the research conducted thus far
- Enforcing Good Laboratory Practices (GLP) to ascertain researchers’ compliance to relevant guidelines
- Reporting any significant non-conformance to animal use protocols and withdrawing the ethical approval for these studies, if needed
What do federal guidelines for ethical research on animals include?
Typically, all the animal research guidelines issued by governmental bodies involve a comprehensive set of rules. Knowing these will help you prepare for your research on animals.
Health status of the animals and their veterinary care |
Quarantine, separation, and stabilization of sick animals |
Surveillance, diagnosis, treatment and control of disease among animals |
The physical condition and design of the animal facility including humidity, noise, and temperature control; lighting, ventilation; caging and housing system; separate areas for animal breeding; sheltered, outdoor housing with activity areas for non-human primates, dogs, birds, etc. |
Specialized laboratories for experiments involving intensive care, hazardous chemicals, necropsy, radiography, preparation of special diets, experimental manipulation, treatment and diagnostic laboratory procedures, and containment facilities |
Separate areas for washing, autoclaving/sterilization, and storage of food and bedding |
Stipulations on the types of bedding and quality of feed and water used |
Transport of animals |
Duration of experiments |
Use of physical restraint to hold the animals during experiments |
Animal experimentation involving hazardous agents, recombinant DNA/vaccine and/or samples from human origin |
Use of anaesthesia and euthanasia |
Multiple surgical procedures on a single animal |
Use and care of transgenic animals and nude mice |
Rules on breeding the animals |
Care of animals on holidays, weekends, and emergencies |
Training of technical persons for the care of animals as well as showers, lockers, sinks, and toilets for these personnel |
Waste disposal and assessment of the quality of sanitation |
Pest control |
Standard operating procedures (SOPs) for all the people using the animal facility |
Record keeping |
Visitor and security policies |
That brings us to the end of this generic post on federal bodies stipulating regulations for the care and ethical treatment of animals used in research. In the next segment of this series, I’ll focus on what these regulatory bodies do and how they assess applications.
Here’s an exercise for you
If you are conducting research on animals, check if your institution and country have instituted regulatory bodies for monitoring animal research. Go ahead and take a look at what their regulations include. Try and get a sense of the things you need to remember when conducting your research.
Related reading:
- US Government Publishing Office Food and Drug Regulations
- Guidelines on Good Clinical Laboratory Practice
- Animal use in pharmacology education and research: The changing scenario
- A young researcher's guide to a clinical trial
- Can a veterinary medicine paper be published without informed consent of animal owner?
Other parts in the series:
Published on: Feb 22, 2018
Comments
You're looking to give wings to your academic career and publication journey. We like that!
Why don't we give you complete access! Create a free account and get unlimited access to all resources & a vibrant researcher community.

Subscribe to Conducting Research













