|
Getting your Trinity Audio player ready...
|
Biomedical research involves the use of various study designs to investigate health and disease. Understanding the different types of study designs is crucial for interpreting and evaluating research findings. Research designs are broadly divided into observational studies (i.e., cross-sectional; case–control and cohort studies) and experimental studies (randomized control trials, certain basic science studies). Here’s a brief explanation of the most common study designs used in biomedical research:
Basic Science Studies
Basic science studies are laboratory-based studies that aim to shed light on the underlying mechanisms of a disease or condition. Basic science studies are often conducted in animal models or cell cultures and focus on the biological basis of a disease at a molecular or cellular level.
Randomized Controlled Trials
Randomized controlled trials (RCTs) are considered the gold standard in evaluating the effectiveness of an intervention or treatment, especially newly developed treatments. They involve randomly assigning participants to either a treatment group or a control group. This design is used to test the efficacy of interventions and to determine cause-and-effect relationships between variables. RCTs are conducted in different phases:
Phase 0: Exploring if and how a new treatment works
Phase I: Finding the highest dose of the new treatment that can be given safely without severe side effects
Phase II: Investigating whether the treatment works for a larger number of patients
Phase III: Determining whether the treatment is better than what’s already available
Phase IV: Examining other questions, such as the effect on longevity and quality of life, or cost-effectiveness
Cohort Studies
Cohort studies follow a group of individuals over a period of time to determine the incidence of disease or risk factors for disease. Cohort studies can be prospective or retrospective. They are used to investigate the natural history of the disease, identify risk factors, and evaluate the effectiveness of interventions.
Case-Control Studies
Case-control studies are retrospective studies that compare individuals with a particular disease (i.e., cases) to those without the disease (i.e., controls) in order to identify potential risk factors. This design is useful for investigating rare diseases or diseases with long latency periods.
Cross-Sectional Studies
Cross-sectional studies collect data at a single point in time. They are used to determine the prevalence of a particular disease or risk factor at that time. This design is useful for identifying patterns of disease and for generating hypotheses.
Retrospective Chart Reviews
Retrospective chart reviews involve examining medical records to extract data about a particular disease or condition. This type of study is often used to investigate the epidemiology of a disease, identify risk factors, and evaluate the effectiveness of interventions.
Systematic Reviews and Meta-Analyses
Systematic reviews and meta-analyses are used to synthesize the results of multiple studies on a particular topic and to provide a consolidated view of existing evidence. This design is useful for evaluating the overall strength of the evidence, especially across countries and time periods, and for identifying areas where further research is needed.
How to choose your Study Design
The quality of your research and the evidence it provides can be greatly affected by your choice of study design. For instance, longitudinal evidence is considered stronger than cross-sectional evidence. The infographic below outlines what you need to consider when choosing your study design.
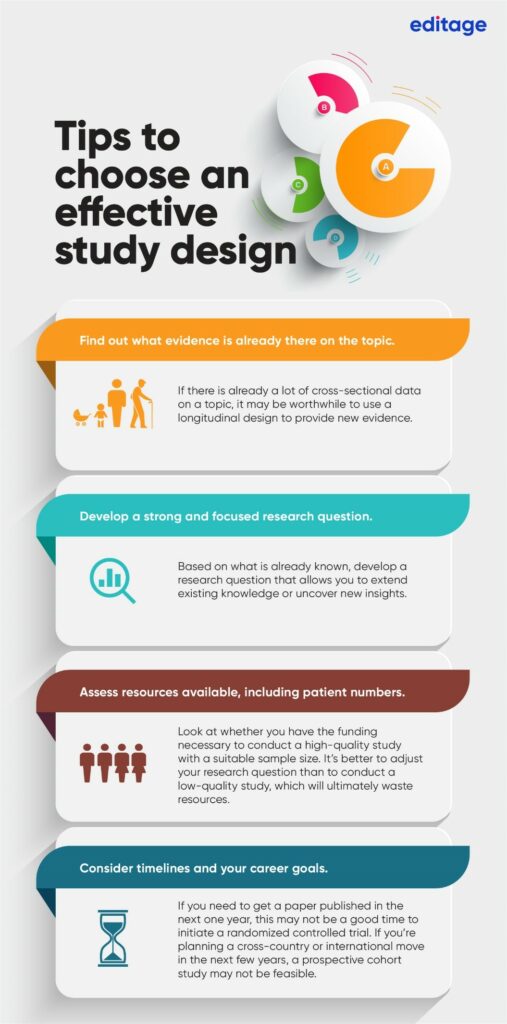
Each study design has its strengths and limitations. Understanding the different types of study designs can help you design studies that are rigorous and can generate useful evidence, contributing to the overall knowledge.
If you’re looking for an expert statistical analysis service to support you in designing a high-quality study, book a conversation with our consultant today.


Comment